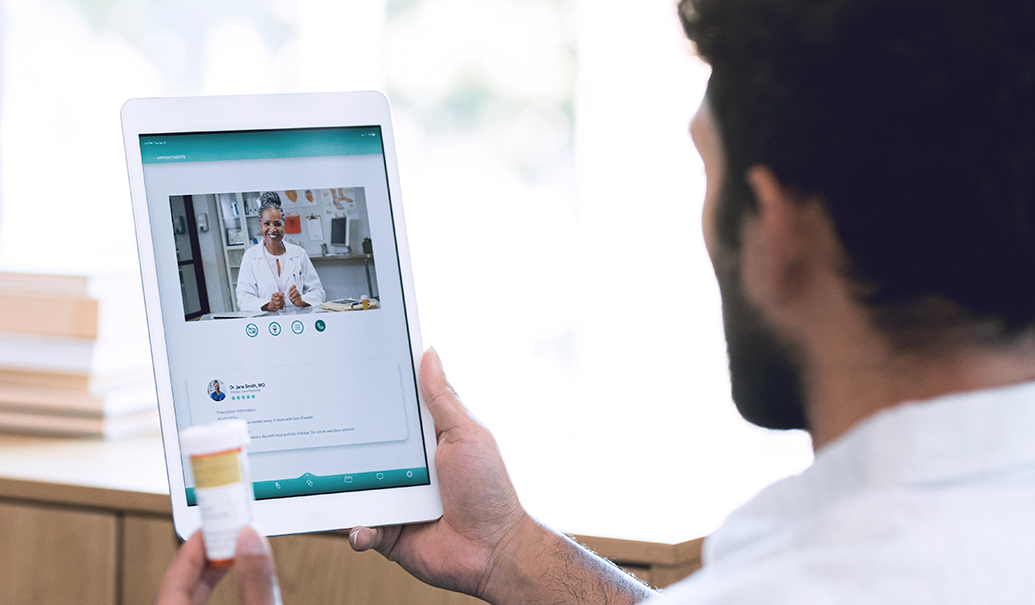
Many life sciences companies strive to improve patient access through better connectivity to telehealth options. Learn about the various compliance and design considerations that life sciences companies should evaluate to launch successful telehealth offerings, known as the digital front door. In this webinar from the 2022 Annual Pharmaceutical and Medical Device Ethics and Compliance Congress, ZS’s head of global compliance and privacy, Michael Shaw, and ZS consultant, Rita Pan, are joined by Sidley Austin’s healthcare practice lead, Meenakshi Datta, to share key strategies that will help life sciences companies meet their digital business priorities while navigating compliance risks.
Watch the webinar to learn:
- Key objectives for telehealth connectivity platforms and compliance considerations
- Strategies for integrating compliance controls into life sciences telehealth frameworks
- Practical considerations to align telehealth platform design with operational objectives and compliance standards
- Key success factors to support successful telehealth platform launches
Add insights to your inbox
We’ll send you content you’ll want to read – and put to use.