Solutions for life sciences
ZS offers a unique perspective on how to bring business impact to the science of R&D. Our strategy and life sciences consulting team works with business executives to align their R&D vision, benchmark capabilities, develop their R&D strategy, design organizational capabilities and lead strategic initiatives.

Accelerating drug development
Using digital science to advance medicine
Unlocking the insights beyond the surface
Watch our video on how we’re accelerating R&D with data and analytics.
Why ZSers are excited about R&D
Leaders in life sciences research and development at ZS share their thoughts on the issues that inspire their passion and why this is an exciting time for their industry.
- Section container 1
- Section container 2
LIFE SCIENCES CONSULTING
R&D & Medical Solutions
Partner with ZS for deep life sciences, information science and management science expertise, and end-to-end solutions for your most challenging scientific opportunities.

ZS Trials.AI
Integrated evidence strategy and planning
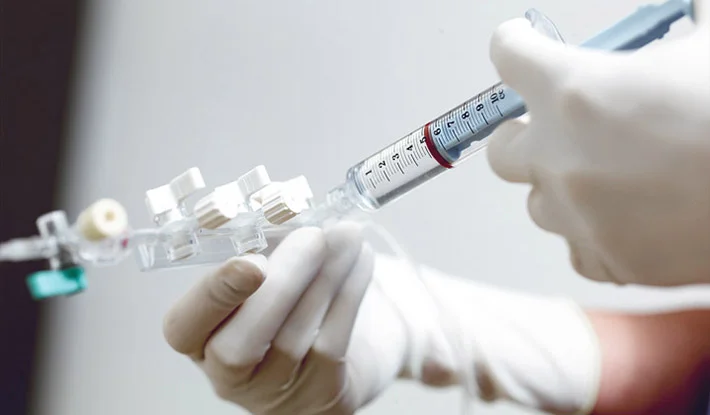
Clinical Development

Medical Affairs

ZS Discovery

Value & Access

Reimagine clinical development with in silico trials
Learn how leading companies such as Roche, AstraZeneca, Pfizer and BMS are pioneering in silico methods across the clinical development life cycle to drive smarter, faster clinical trials. Explore use cases, examples from top pharma companies and a set of modular in silico capabilities ready to scale across the clinical trial life cycle. The era of simulation-first trials has arrived.

The future of drug development has arrived
The data, technology and appetite to transform clinical development are here. It’s time to build an operating model that eliminates guesswork, removes barriers and delivers therapeutic innovation faster and at lower cost. Explore four imperatives to digitally transform your clinical trials.
- 1
- 2
Insights
Explore the full ecosystem
ZS has partnered with these industry-leading organizations to deliver the highest quality services and solutions to our clients.