Clinical Development
Helping sponsors, sites and patients enhance the impact of clinical trials through the “head and heart,” combining technology, data and analytics with the human experience to accelerate change in clinical research.
WHAT WE DO
Challenges we solve in clinical trials
Digital and technology transformation
You need to develop technology that’s adaptable to any IT ecosystem and design products where scale and speed to innovation have the greatest impact.
Unlocking the potential of AI in clinical trials
You need the expertise to help clinical leaders plan, enhance and accelerate the AI quotient in the sponsor’s clinical research organization and function.
Portfolio strategy
The lightning-fast pace of change in clinical development makes portfolio strategy more important than ever. A sharpened focus on patient experience and the integration of virtual technology into trials requires careful thought and planning.
Protocol design
Data and competitive intelligence exist in silos, which presents formidable hurdles when prioritizing portfolio investments and determining optimal study design.
Program planning
At a global scale, how can you predict the success of your clinical trials? It can be difficult to engage the right sites and investigators to ensure that you’re mitigating risk, maintaining quality and ensuring patient engagement.
Study execution and data collection
You need a global and holistic picture of how your data is organized. You also need to identify any drivers that shift study performance and real-time data to make necessary modifications.
HOW WE DO IT
ZS’s approach
We partner with R&D leaders and clinical research organizations to improve clinical development solutions through thoughtful design, trial differentiation and predictive modeling. We’re delivering greater value to patients and transforming the drug pipeline.
Trial design optimization
Facilitate collaboration with our Clinical Design Center, a virtual environment that allows cross-functional teams to work together on clinical trial design powered by AI to speed trial program planning and execution.
Quality and clinical operations risk management
Identify, monitor and predict clinical development risk with ZS’s integrated quality, operations and risk management (QORM) offerings.
Human-centric clinical trials
Accelerate planned and in-flight studies through a strategic evaluation of trial stakeholders (investigators, study coordinators and patients) to understand what they value in a study and how to engage them in the most impactful way.
Biometrics and clinical data solutions
Overhaul legacy infrastructure and processes to accelerate clinical data management, biostatistics and programming capabilities.
Digital and decentralized trials
Get support through an ecosystem of solutions and services while transforming your clinical trial programs to match today’s trends.
Trial participant diversity, equity and inclusion
Improve diversity, equity and inclusion in clinical development through a comprehensive approach built on a foundation of patient insights.

Life sciences R&D & Medical
Enable your AI with the blueprint for Data at the Speed of Light
Seamless, standards-driven data flow in clinical trials is now within our reach. In this article, we give real-world examples of how major pharma companies such as Bayer, Roche and Novo Nordisk are building standards-first data pipelines, where protocols are structured once and then flow seamlessly from design through execution and submission. We also lay out a blueprint for you to enable Data at the Speed of Light in your own organization.

Reimagine clinical development with in silico trials
Learn how leading companies such as Roche, AstraZeneca, Pfizer and BMS are pioneering in silico methods across the clinical development life cycle to drive smarter, faster clinical trials. Explore use cases, examples from top pharma companies and a set of modular in silico capabilities ready to scale across the clinical trial life cycle. The era of simulation-first trials has arrived.

LIFE SCIENCES R&D & MEDICAL
The future of drug development has arrived
The data, technology and appetite to transform clinical development are here. It’s time to build an operating model that eliminates guesswork, removes barriers and delivers therapeutic innovation faster and at lower cost. Explore four imperatives to digitally transform your clinical trials.
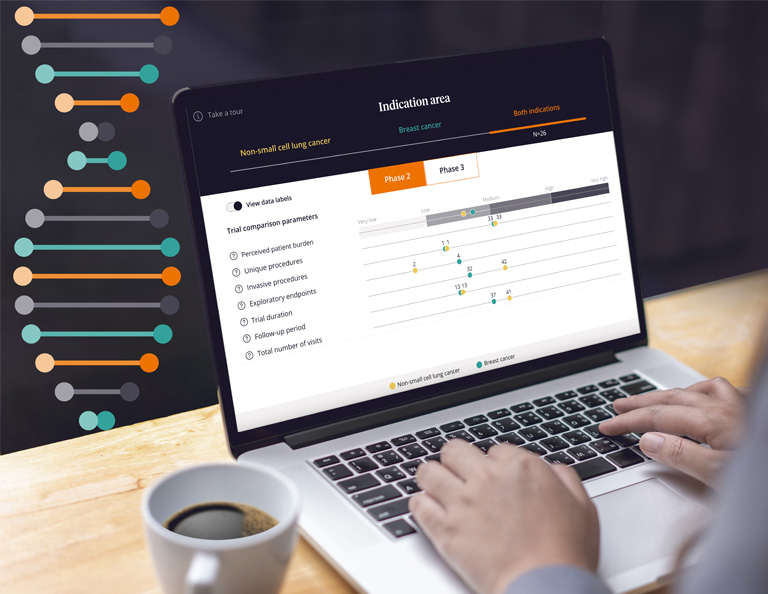
LIFE SCIENCES R&D & MEDICAL
Transform clinical trial design
Clinical trials are complex. That’s why we’ve developed a quantifiable, repeatable method to visualize and optimize trial design. See how your company can enhance the speed, cost and quality of your trials.

Pharmaceuticals & Biotech
Getting the clinical trial site perspective
On this episode of “Transforming Biopharma,” Maria Whitman talks with site experts Lauren Wall of the University of Chicago and Caroline Smith of Charter Research about how pharma can forge successful partnerships.

R&D INNOVATION
Unlocking a wealth of insights
Together with life sciences leaders, we are paving the way to new innovations and faster cures, using the power of data to find patient-centered solutions that make a real impact.
- 1
- 2
- 3
- 4
- 5
- 6
Connect with our experts
Related clinical offerings
ZAIDYN Clinical Development
Identify high-risk sites, enhance recruitment and site engagement, predict trial enrollment and provide teams with critical study planning insights.

Trials.AI
Leverage AI, tech and deep expertise for smarter study design, optimized participant experience and faster time to market.
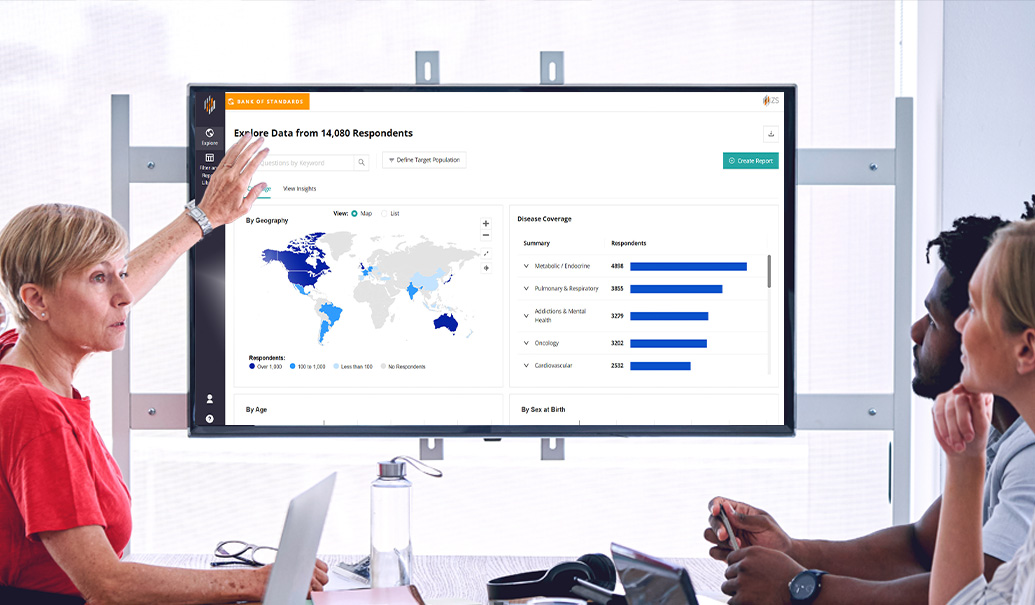
ZS Patient Experience Bank
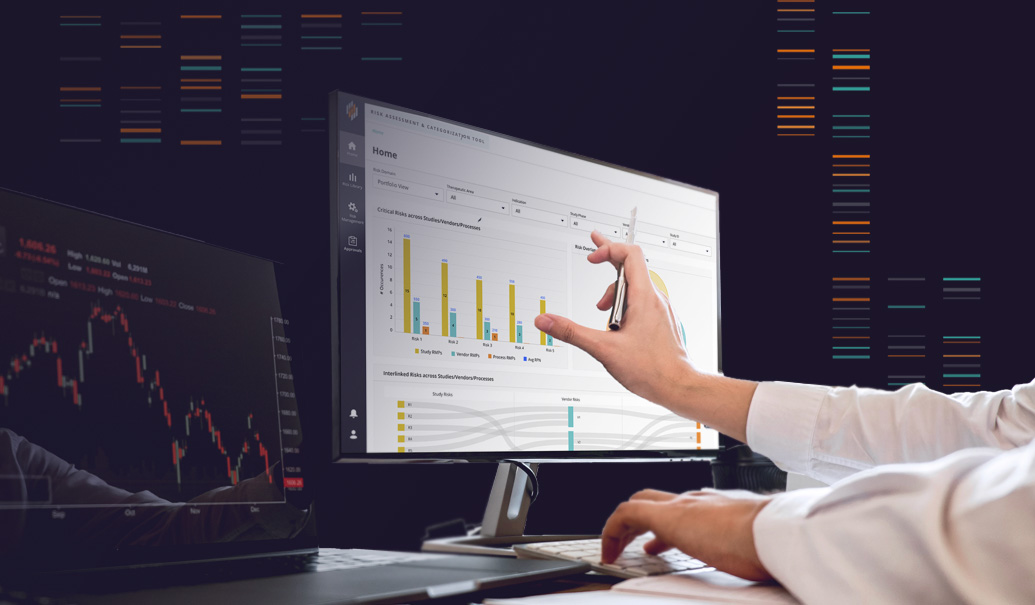
ZS RBQM360
A unique and flexible solution that enables portfolio and study-level RBQM, providing sponsors with enhanced quality, speed and cost efficiency.

Biometrics
Improve legacy infrastructure and processes to accelerate clinical data management, biostatistics and programing capabilities.

Scaled Patient Voice
A centralized platform to scale, operationalize and seamlessly integrate patient insights to drive effective recruitment and retention strategies.
Featured insights
In the news
Life Sciences R&D & Medical
Study shows industry consensus needed in attitudes toward DCT quality risks
Sept. 12, 2022 | Article
Related solutions

Life Sciences R&D & Medical

Biopharmaceutical Statistics Leaders Consortium
Integrated evidence strategy and planning

Medical Affairs

ZS Discovery
















